16 3.3 Biochemical Compounds
Created by: CK-12/Adapted by Christine Miller
The original version of this chapter contained H5P content. You may want to remove or replace this element.
Figure 3.3.1 Carbo-licious!
Carbs Galore
What do all of these foods have in common? All of them consist mainly of large compounds called carbohydrates, often referred to as “carbs.” Contrary to popular belief, carbohydrates are an important part of a healthy diet. They are also one of four major classes of biological macromolecules.
Chemical Compounds in Living Things

The compounds found in living things are known as biochemical compounds or biological molecules. Biochemical compounds make up the cells and other structures of organisms. They also carry out life processes. Carbon is the basis of all biochemical compounds, so carbon is essential to life on Earth. Without carbon, life as we know it could not exist.
Carbon is so basic to life because of its ability to form stable bonds with many elements, including itself. This property allows carbon to create a huge variety of very large and complex molecules. In fact, there are nearly 10 million carbon-based compounds in living things!
Most biochemical compounds are very large molecules called polymers. A polymer is built of repeating units of smaller compounds called monomers. Monomers are like the individual beads on a string of beads, and the whole string is the polymer. The individual beads (monomers) can do some jobs on their own, but sometimes you need a larger molecule, so the monomers can be connected to form polymers.
Classes of Biochemical Compounds
Although there are millions of different biochemical compounds in Earth’s living things, all biochemical compounds contain the elements carbon, hydrogen, and oxygen. Some contain only these elements, while others contain additional elements, as well. The vast number of biochemical compounds can be grouped into just four major classes: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates
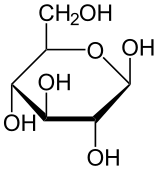
Carbohydrates include sugars and starches. These compounds contain only the elements carbon, hydrogen, and oxygen. In living things, carbohydrates provide energy to cells, store energy, and form certain structures (such as the cell walls of plants). The monomer that makes up large carbohydrate compounds is called a monosaccharide. The sugar glucose, represented by the chemical model in Figure 3.3.2, is a monosaccharide. It contains six carbon atoms (C), along with several atoms of hydrogen (H) and oxygen (O). Thousands of glucose molecules can join together to form a polysaccharide, such as starch.
Lipids

Lipids include fats and oils. They primarily contain the elements carbon, hydrogen, and oxygen, although some lipids contain additional elements, such as phosphorus. Lipids function in living things to store energy, form cell membranes, and carry messages. Lipids consist of repeating units that join together to form chains called fatty acids. Most naturally occurring fatty acids have an unbranched chain of an even number (generally between 4 and 28) of carbon atoms.
Proteins

Proteins include enzymes, antibodies, and many other important compounds in living things. They contain the elements carbon, hydrogen, oxygen, nitrogen, and sulfur. Functions of proteins are very numerous. They help cells keep their shape, compose muscles, speed up chemical reactions, and carry messages and materials. The monomers that make up large protein compounds are called amino acids. There are 20 different amino acids that combine into long chains (called polypeptides) to form the building blocks of a vast array of proteins in living things.
Nucleic Acids
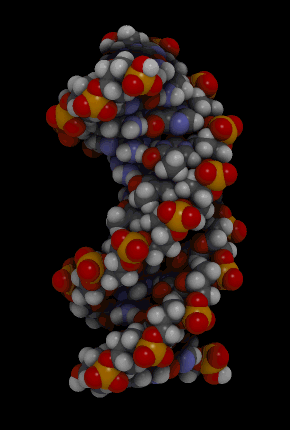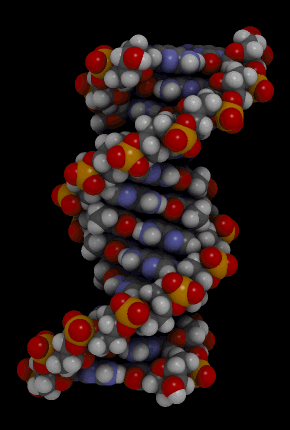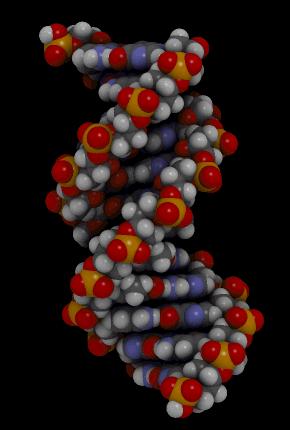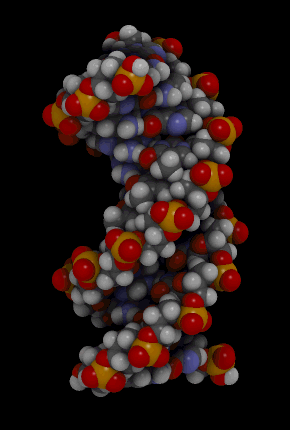
Nucleic acids include the molecules DNA (deoxyribonucleic acid) and RNA(ribonucleic acid). They contain the elements carbon, hydrogen, oxygen, nitrogen, and phosphorus. Their functions in living things are to encode instructions for making proteins, to help make proteins, and to pass instructions between parents and offspring. The monomer that makes up nucleic acids is the nucleotide. All nucleotides are the same, except for a component called a nitrogen base. There are four different nitrogen bases, and each nucleotide contains one of these four bases. The sequence of nitrogen bases in the chains of nucleotides in DNA and RNA makes up the code for protein synthesis, which is called the genetic code. The animation in Figure 3.3.5 represents the very complex structure of DNA, which consists of two chains of nucleotides.
3.3 Summary
- Biochemical compounds are carbon-based compounds found in living things. They make up cells and other structures of organisms and carry out life processes. Most biochemical compounds are large molecules called polymers that consist of many repeating units of smaller molecules, which are called monomers.
- There are millions of biochemical compounds, but all of them fall into four major classes: carbohydrates, lipids, proteins, and nucleic acids.
- Carbohydrates include sugars and starches. They provide cells with energy, store energy, and make up organic structures, such as the cell walls of plants.
- Lipids include fats and oils. They store energy, form cell membranes, and carry messages.
- Proteins include enzymes, antibodies, and numerous other important compounds in living things. They have many functions — helping cells keep their shape, making up muscles, speeding up chemical reactions, and carrying messages and materials.
- Nucleic acids include DNA and RNA. They encode instructions for making proteins, help make proteins, and pass encoded instructions from parents to offspring.
3.3 Review Questions
- Why is carbon so important to life on Earth?
- What are biochemical compounds?
- Describe the diversity of biochemical compounds and explain how they are classified.
- Identify two types of carbohydrates. What are the main functions of this class of biochemical compounds?
- What roles are played by lipids in living things?
- The enzyme amylase is found in saliva. It helps break down starches in foods into simpler sugar molecules. What type of biochemical compound do you think amylase is?
- Explain how DNA and RNA contain the genetic code.
- What are the three elements present in every class of biochemical compound?
- Classify each of the following terms as a monomer or a polymer:
- Nucleic acid
- Amino acid
- Monosaccharide
- Protein
- Nucleotide
- Polysaccharide
- Match each of the above monomers with its correct polymer and identify which class of biochemical compound is represented by each monomer/polymer pair.
- Is glucose a monomer or a polymer? Explain your answer.
- What is one element contained in proteins and nucleic acids, but not in carbohydrates?
- Describe the relationship between proteins and nucleic acids.
- Why do you think it is important to eat a diet that contains a balance of carbohydrates, proteins, and fats?
- Examine the picture of the meal in Figure 3.3.6. What types of biochemical compounds can you identify?

3.3 Explore More
Biomolecules (updated), by the Amoeba Sisters, 2016.
Attributions
Figure 3.3.1
- Cinnamon buns by adamkontor on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
- Sugar by Bru-nO on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
- Potatoes by HolgersFotografie on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
- Blueberries; oatmeal by iha31 on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
- Bread by pics_pd on Pixnio is used under a public domain certification (https://creativecommons.org/licenses/publicdomain/).
- Spaghetti by RitaE on Pixabay is used under the Pixabay License (https://pixabay.com/de/service/license/).
Figure 3.3.2
jewellery_beads_stones_necklace-1200668 on Pxhere, is used under a CC0 1.0 universal public domain dedication license (https://creativecommons.org/publicdomain/zero/1.0/).
Figure 3.3.3
Glucose; Structure of beta-D-glucopyranose (Haworth projection), by NEUROtiker on Wikimedia Commons, has been released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.3.4
Lipid Examples; Butter and Oil, by Bill Branson (photographer), on Wikimedia Commons is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.3.5
Protein-rich_Foods, by Smastronardo on Wikimedia Commons, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
Figure 3.3.6
Bdna_cropped [gif], by Jahobr on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain) (This is a derivative work from Bdna.gif by Spiffistan.)
Figure 3.3.7Dinner by Quốc Trung [@boeing] on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Reference
Amoeba Sisters. (2016, February 11). Biomolecules (updated). YouTube. https://www.youtube.com/watch?v=YO244P1e9QM&feature=youtu.be
A biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1. Complex carbohydrates are polymers made from monomers of simple carbohydrates, also termed monosaccharides.
A very large molecule, such as protein, commonly created by the polymerization of smaller subunits (monomers).
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
A large molecule, or macromolecule, composed of many repeated subunits.
A molecule that can undergo polymerization, creating macromolecules. Large numbers of monomers combine to form polymers in a process called polymerization.
A substance that is insoluble in water. Examples include fats, oils and cholesterol. Lipids are made from monomers such as glycerol and fatty acids.
A class of biological molecule consisting of linked monomers of amino acids and which are the most versatile macromolecules in living systems and serve crucial functions in essentially all biological processes.
A complex organic substance present in living cells, especially DNA or RNA, whose molecules consist of many nucleotides linked in a long chain.
The ability to do work.
Biological molecules that lower amount the energy required for a reaction to occur.
Image shows a diagram of the three types of RNA: Messenger RNA, which is a single strand of RNA, Ribosomal RNA, which is an RNA-protein complex with two subunits, and transfer RNA, which is a single strand of RNA enfolded on itself with an anticodon region and a region which can carry a single amino acid.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another.
Amino acids are organic compounds that combine to form proteins.
Deoxyribonucleic acid - the molecule carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses.
A nucleic acid of which many different kinds are now known, including messenger RNA, transfer RNA and ribosomal RNA.

