24 3.11 Water and Life
Created by: CK-12/Adapted by Christine Miller

The Blue Marble
It’s often called the “water planet,” and it’s been given the nickname “the blue marble.” You probably just call it “home.” Almost three-quarters of our home planet is covered by water, and without it, life as we know it could not exist on Earth. Water, like carbon, has a special role in living things: it is needed by all known forms of life. Although water consists of simple molecules, each containing just three atoms, its structure gives it unique properties that help explain why it is vital to all living organisms.
Water, Water Everywhere
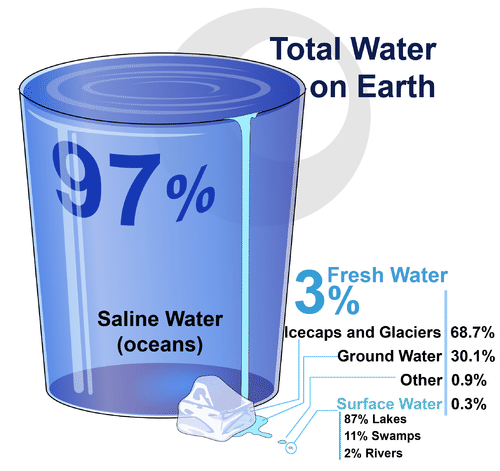
If you look at Figure 3.11.2, you will see where Earth’s water is found. The term water generally refers to its liquid state, and water is a liquid over a wide range of temperatures on Earth. Water, however, also occurs on Earth as a solid (ice) and as a gas (water vapor).
Structure and Properties of Water
You are likely already aware of some of the properties of water. For example, you know that water is tasteless and odorless. You also probably know that water is transparent, which means that light can pass through it. This is important for organisms that live in the water, because some of them need sunlight to make food by photosynthesis.
Chemical Structure of Water
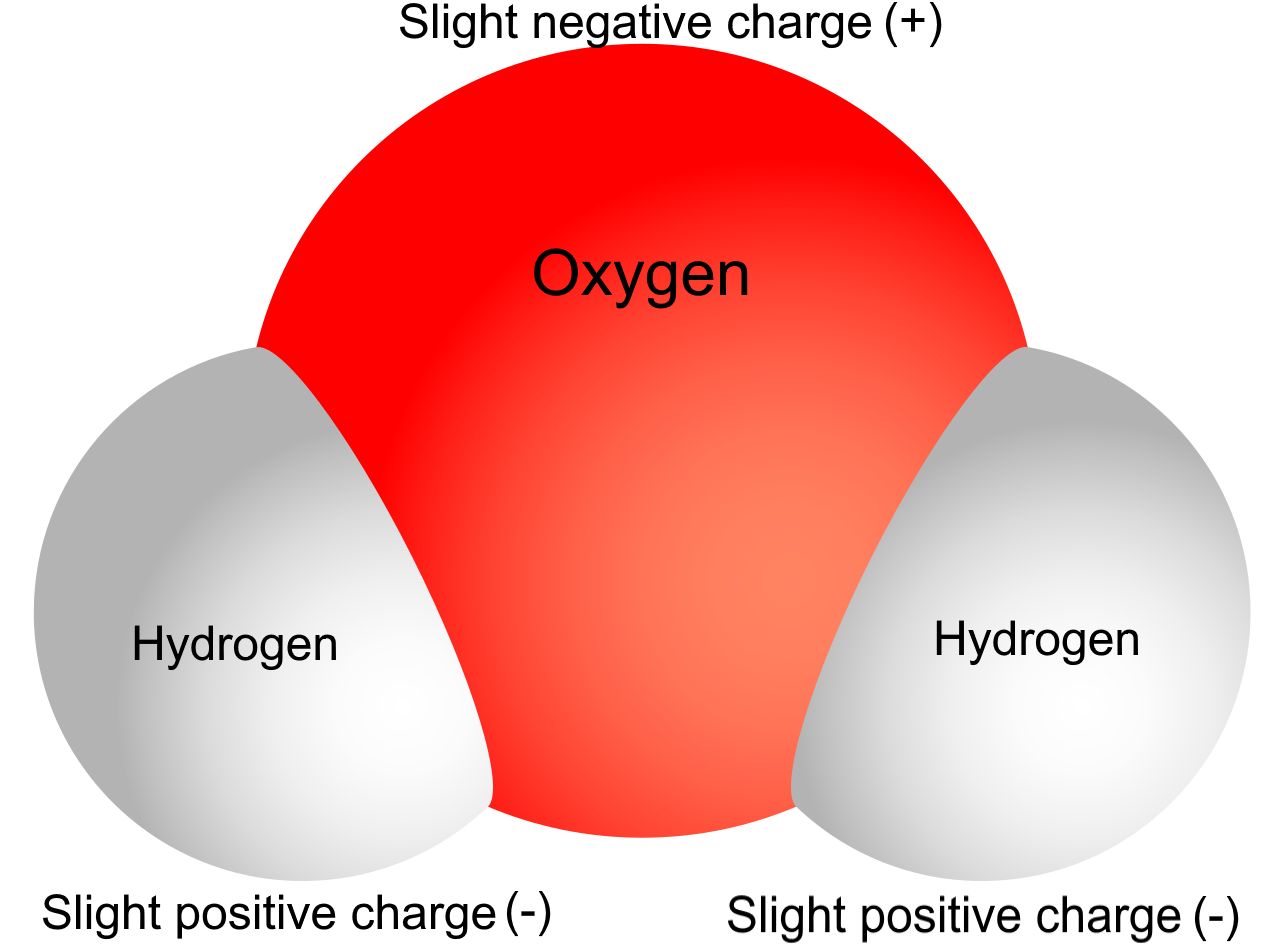
To understand some of water’s properties, you need to know more about its chemical structure. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen. The oxygen atom in a water molecule attracts electrons more strongly than the hydrogen atoms do. As a result, the oxygen atom has a slightly negative charge, and the hydrogen atoms have a slightly positive charge. A difference in electrical charge between different parts of the same molecule is called polarity. The diagram in Figure 3.11.3 shows water’s polarity.
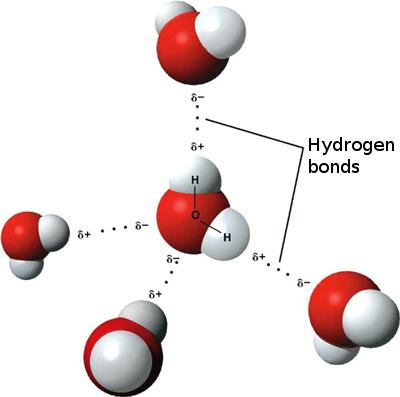
When it comes to charged molecules, opposites attract. In the case of water, the positive (hydrogen) end of one water molecule is attracted to the negative (oxygen) end of a nearby water molecule. Because of this attraction, weak bonds form between adjacent water molecules, as shown in Figure 3.11.4. The type of bond that forms between water molecules is called a hydrogen bond. Bonds between molecules are not as strong as bonds within molecules, but in water, they are strong enough to hold together nearby molecules.
How do you think hydrogen bonding affects water’s properties?
Properties of Water

Hydrogen bonds between water molecules explain some of water’s properties — for example, why water molecules tend to “stick” together. Did you ever watch water drip from a leaky faucet or from a melting icicle? If you did, then you know that water always falls in drops, rather than as separate molecules. The dew drops pictured to the left are another example of water molecules sticking together.
Hydrogen bonds cause water to have a relatively high boiling point of 100°C (212°F). Extra energy is needed to break these bonds and separate water molecules so they can escape into the air as water vapor. Because of its high boiling point, most water on Earth is in a liquid state, rather than a gaseous state. Water in its liquid state is needed by all living things. Hydrogen bonds also cause water to expand when it freezes. This, in turn, causes ice to have a lower density (that is, less mass per unit volume) than liquid water. The lower density of ice means that it floats on water. In cold climates, ice floats on top of the water in lakes. This allows lake animals like fish to survive the winter by staying in the liquid water under the ice.
Watch the video below to hear more about hydrogen bonding and it’s effects on the properties of water:
Why does ice float in water? – George Zaidan and Charles Morton, TED-ED, 2013.
Water and Living Things
The human body is about 70 per cent water (not counting the water in body fat, which varies from person to person). The body needs all this water to function normally. Just why is so much water required by human beings and other organisms? Water can dissolve many substances that organisms need. Water’s polarity helps it dissolve other polar substances. Water is also necessary for many biochemical reactions. The examples below are among the most important biochemical processes that occur in living things, but they are just two of the many ways that water is involved in biochemical reactions.
- Photosynthesis: In this process, cells use the energy in sunlight to change carbon dioxide and water to glucose and oxygen. The reactions of photosynthesis can be represented by the chemical equation:
6CO2 + 6H2O + Energy → C6H12O6 + 6O2
- Cellular respiration: In this process, cells break down glucose in the presence of oxygen and release carbon dioxide, water, and energy. The reactions of cellular respiration can be represented by the chemical equation:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Water is involved in many other biochemical reactions and almost all life processes depend on water.
Feature: My Human Body

Are you a marathon runner or other endurance athlete? Do you live and work in a hot, humid climate? If you answered “yes” to either question, you may be at risk of water intoxication.
Water is considered the least toxic chemical compound, so it may surprise you to learn that drinking too much water can cause serious illness and even death. Water intoxication is a potentially fatal disturbance in brain functions. It results when the normal balance of sodium and other electrolytes in the body is pushed outside safe limits by overhydration, or taking in too much water. The condition is also called hyponatremia, which refers to a lower-than-normal level of sodium in the blood that occurs when more water is entering than leaving the body.
As excessive water is consumed, fluid outside the cells decreases in its concentration of sodium and other electrolytes relative to the concentration inside the cells. This causes fluid to enter the cells by osmosis to balance the electrolyte concentration. The extra fluid in the cells causes them to swell. In the brain, this swelling increases the pressure inside the skull. It is this increase in pressure that leads to the first observable symptoms of water intoxication, which typically include headache, confusion, irritability, and drowsiness. As the condition worsens, additional symptoms may occur, such as difficulty breathing during exertion, muscle weakness and pain, or nausea and vomiting. If the condition persists, the cells in the brain may swell to the point where blood flow is interrupted or pressure is applied to the brain stem. This is extremely dangerous and may lead to seizures, brain damage, coma, or even death.
Under normal circumstances, it is very rare to accidentally consume too much water. However, it is relatively common in athletes who participate in endurance activities, such as marathon running. A study conducted on participants of the 2002 Boston Marathon, for example, found that 13 per cent of the runners finished the race with water intoxication (Almond, et al., 2005). The study also found that water intoxication was just as likely to occur in runners who drank sports drinks containing electrolytes as those who drank plain water. Water intoxication is so common at marathon events that medical personnel who work at such events are trained to suspect water intoxication when runners collapse or show signs of confusion.
Because of the publicity water intoxication has received lately, sports experts have lowered their recommendations for water intake during endurance events. They now advise drinking only when thirsty rather than drinking to “stay ahead of thirst,” which they recommended previously. Keeping water intake in line with water loss is the best way to prevent water intoxication. Mild water intoxication can be treated by restricting fluid intake. In more severe cases, treatment may require the use of diuretic drugs (which increase urination) or other types of drugs to reduce blood volume. Serious water intoxication should be considered a true medical emergency.
3.11 Summary
- Most water on Earth consists of salt water in the oceans. Only a tiny percentage of the Earth’s water is fresh liquid water.
- Virtually all living things on Earth require liquid water. Water exists as a liquid over a wide range of temperatures and dissolves many substances. These properties depend on water’s polarity, which causes water molecules to “stick” together.
- The human body is about 70 per cent water (outside of fat). Organisms need water to dissolve many substances and for most biochemical processes, including photosynthesis and cellular respiration.
3.11 Review Questions
- Where is most of Earth’s fresh water found?
- Identify properties of water.
- What is polarity? Explain why water molecules are polar.
- Why do water molecules tend to “stick” together?
- What role does water play in photosynthesis and cellular respiration?
- Which do you think is stronger: the bonds between the hydrogen and oxygen atoms within a water molecule, or the bonds between the hydrogen and oxygen atoms between water molecules? Explain your answer.
- Given what you’ve learned about water intoxication (or hyponatremia), explain why you think drinking salt water would be bad for your cells.
- What is the name for the bonds that form between water molecules?
- Explain why water can dissolve other polar molecules.
- If there is pollution in the ocean that causes the water to become more cloudy or opaque, how do you think the ocean’s photosynthetic organisms will be affected? Explain your answer.
- Describe one way in which your body gets rid of excess water.
- True or False: Ice floats on top of water because it is denser than water.
3.11 Explore More
Properties of Water, by The Amoeba Sisters, 2016.
How polarity makes water behave strangely – Christina Kleinberg, TED-Ed, 2013.
Attributions
Figure 3.11.1
Planet Earth by NASA (photo taken by either Harrison Schmitt or Ron Evans (of the Apollo 17 crew), on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.11.2
Total water on earth by LadyofHats at CK12, is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
Figure 3.11.3
Polarity of water by Christine Miller is released into the Public Domain (https://creativecommons.org/publicdomain/mark/1.0/).
Figure 3.11.4
Hydrogen bonds, translated by Michal Maňas (User:snek01) is released into the public domain (https://en.wikipedia.org/wiki/Public_domain). (Original uploader was Qwerter at Czech Wikipedia.)
Figure 3.11.5
Dew by Pascal Chanel on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.11.6
Woman in a wheelchair marathon by Kevin André on Unsplash is used under the Unsplash License (https://unsplash.com/license).
References
Almond, C.S., Shin, A.Y., Fortescue, E.B. et al. (2005, April). Hyponatremia among runners in the Boston Marathon. The New England Journal of Medicine, 352 (15), 1550–1624. doi:10.1056/NEJMoa043901. PMID 15829535.
Amoeba Sisters. (2016, July 26). Properties of Water. YouTube. https://www.youtube.com/watch?v=3jwAGWky98c&feature=youtu.be
Ruiz Villarreal, M. (LadyofHats). (2016, August 15). Figure 2. Total water on earth [digital image]. In Brainard, J., Henderson, R., CK-12’s College Human Biology FlexBook® (section 3.11). CK12 Foundation. https://www.ck12.org/book/ck-12-college-human-biology/
TED-Ed. (2013, February 4). How polarity makes water behave strangely – Christina Kleinberg. YouTube. https://www.youtube.com/watch?v=ASLUY2U1M-8&feature=youtu.be
TED-Ed. (2013, October 22). Why does ice float in water? – George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=UukRgqzk-KE&feature=youtu.be
The smallest particle of an element that still has the properties of that element.
A sub-atomic particle with a charge of -1.
A separation of electric charge leading to a molecule or its chemical groups having a negatively charged end and a positively charged end.
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule.
The ability to do work.
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organisms' activities.
A set of metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP).
A low sodium concentration in the blood often caused by over consumption of water. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance.
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
The movement of water or other solvent through a plasma membrane from a region of low solute concentration to a region of high solute concentration.

